
by admin | Mar 15, 2024 | Language
Clinical trials hold significant importance in the development of medical innovations, acting as the bridge between groundbreaking research and the potential to transform patient care. These trials offer the promise of introducing new devices, treatments, and...

by admin | Feb 19, 2024 | Language
In a 2016 Tufts study, the total cost of introducing a new drug to the market was estimated at around $2.6 billion, with out-of-pocket expenses per approved new compound reaching nearly $1.4 billion. Phase III clinical trials were found to have an average cost of...
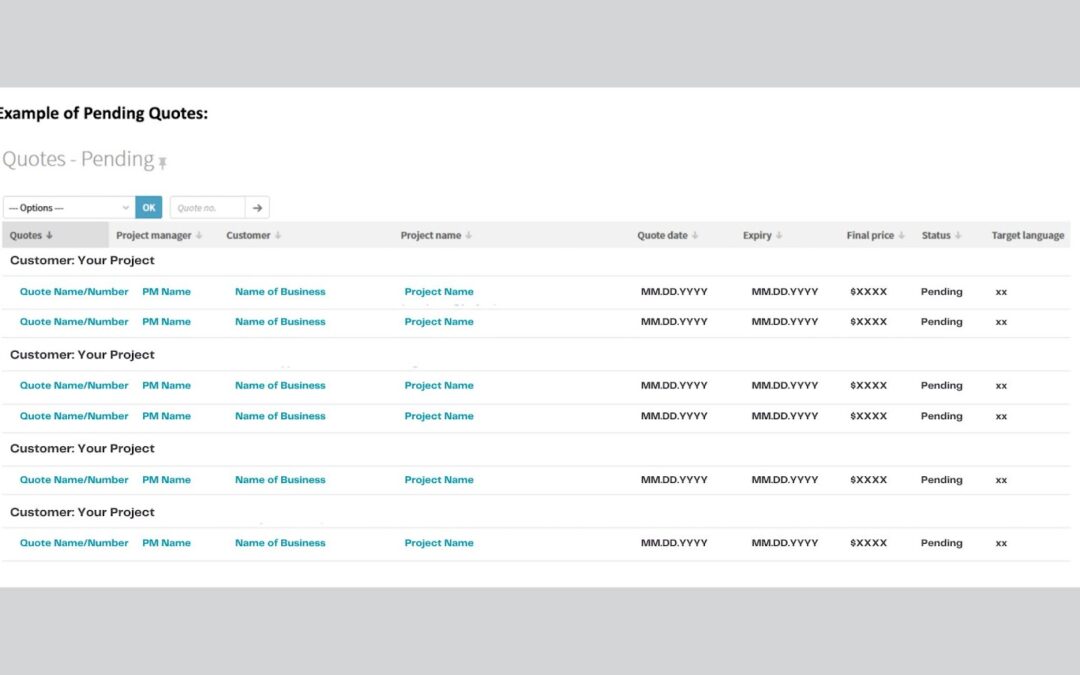
by admin | Jul 6, 2023 | Language
Why You Need a Translation Partner Who Uses Customer Portals to Help You Document translation is a single yet significant step in the clinical trial process. Clinical trials can focus on progress when the process is reliable and straightforward. A critical part of...

by DTS Language Services | Apr 18, 2023 | Clinical Trials Translation, Language
Clinical Trial Translation Mistakes and How to Avoid Them When you’re working on a translation project for a clinical trial, the stakes are high. Of course, the health and safety of patients is a top priority, and accurate translation is a critical piece of that....

by DTS Language Services | Jan 6, 2023 | Language
Are you looking for Norwegian translation services to help streamline your document translations? Norwegian is a complex language, and having the right translation services on hand can make all the difference in ensuring accuracy and efficiency. With our translation...

by DTS Language Services | Dec 12, 2022 | Language
As can be expected, a majority of Israel’s population of 9.3 million speaks native Hebrew. Worldwide, around eight million people grow up speaking Hebrew at home. This makes Hebrew translation vital for all kinds of organizations. And this is especially true in...








